Which Of These Would Not Describe Characteristics Of The Normal Microbiota Of The Skin?
Abstract
The skin microbiota is intimately coupled with cutaneous health and illness. Interactions betwixt commensal microbiota and the multiple prison cell types involved in cutaneous wound healing regulate the immune response and promote barrier restoration. This dialog between host cells and the microbiome is dysregulated in chronic wounds. In this review, we start draw how advances in sequencing approaches and assay have been used to report the chronic wound microbiota, and how these findings underscored the complication of microbial communities and their association with clinical outcomes in patients with chronic wound disorders. We also hash out the mechanistic insights gathered from multiple animate being models of polymicrobial wound infections. In addition to the well-described role of leaner residing in polymicrobial biofilms, we besides talk over the role of the intracellular bacterial niche in wound healing. We describe how, in contrast to pathogenic species capable of subverting skin amnesty, commensals are essential for the regulation of the cutaneous allowed organisation and provide protection from intracellular pathogens through modulation of the antimicrobial molecule, Perforin-2. Despite recent advances, more inquiry is needed to shed light on host-microbiome crosstalk in both healing and nonhealing chronic wounds to appropriately guide therapeutic developments.
FormalPara Key Points
| A complex microbiome is a hallmark of chronic nonhealing wounds. |
| Pathogenic leaner are able to escape skin immunity and even reside inside host cells. |
| Commensal bacteria can modulate the cutaneous immune response to preclude wound infections. |
Introduction
Chronic wounds, the most common of which are diabetic foot ulcers, pressure ulcers, venous leg ulcers, and nonhealing surgical wounds, are a major healthcare trouble. Chronic wounds commonly occur in older individuals with underlying conditions such as diabetes mellitus, vascular disease, and obesity [1]. Compromised immune and nutritional condition, and chronic mechanical stress have also been shown to contribute to poor wound healing [2, 3]. Chronic wounds are associated with alarmingly loftier bloodshed: the 5-yr bloodshed rates of ischemic (55%), neuropathic (45%), and neuroischemic (xviii%) diabetic foot ulcers [4] are college than or similar to those associated with breast cancer and prostate cancer (18% and viii%, respectively) [5]. Chronic wounds are besides associated with high healthcare costs: in the U.s., total spending estimates for chronic nonhealing wounds ranged from U.s.a.$28.1 to US$96.8 billion in 2014 according to a retrospective analysis of the Medicare 5% Limited Data Prepare [6]. Despite the alarming prevalence and high costs of care, efficient treatments are still lacking [2].
Cutaneous wound healing is a complex and very organized procedure, tightly controlled by several cell types through the secretion of growth factors, cytokines, and chemokines as illustrated in Fig. 1 [two]. Disruption of this process prevents the skin bulwark from being properly restored; thus, the wound does not heal and becomes chronic. Higher rates of keratinocyte proliferation, an absence of migration, and fibrosis have all been observed in chronic wounds, regardless of their type. In contrast to the normal process of wound healing, angiogenesis, stem prison cell recruitment and activation, and extracellular matrix remodeling accept all been shown to be impaired in chronic wounds, whereas inflammation has been found to exist persistent and unresolved [two, 7,8,9,ten,11]. Although the pathology of wound healing is now well characterized, the cellular and molecular mechanisms of dumb wound healing are yet being studied. In particular, the role of microorganisms in chronic wound pathology is non fully understood and the importance of topical antimicrobial agents in their treatment is continuously debated.
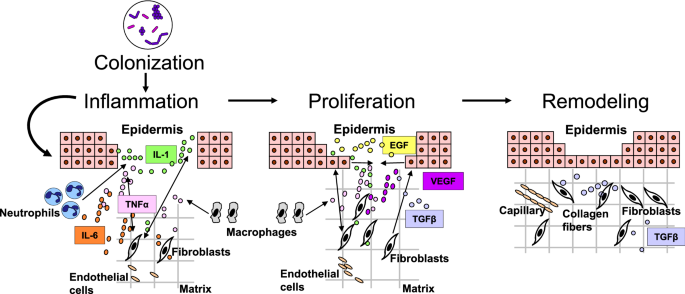
Cutaneous wound healing progression: overlapping phases of acute healing. Epidermal keratinocytes, neutrophils, and macrophages are major prison cell types involved in the inflammatory response that occurs concurrently with hemostasis in the early on stages of wound healing. Colonization of wounds with commensal microbiota may promote wound healing through activation of the innate immune response. In the proliferation phase, keratinocytes multiply and migrate, fibroblasts drift and deposit extracellular matrix, and angiogenesis occurs. Extracellular matrix remodeling results in scar formation and the ultimate restoration of the peel barrier. The most common growth factors and cytokines are shown. EGF epidermal growth cistron, IL interleukin, TGFβ transforming growth cistron-beta, TNFα tumor necrosis cistron-alpha, VEGF vascular endothelial growth factor
Wounds provide an opportunity for microorganisms from the peel surface that found the skin microbiota, coupled with those from the environment, to gain entry to the underlying tissues and find optimal conditions for colonization and growth [12, thirteen]. Interaction of commensal microorganisms with the skin cells during the normal cutaneous wound healing procedure is thought to exist beneficial in modulating the innate immune response [12, 14, fifteen]. Conversely, pathogenic microorganisms are suspected to play a substantial role in delayed wound healing [two, sixteen]. Thus, analyzing skin microbiota composition in both the normal astute and impaired wound healing processes is indispensable for the identification of novel therapeutic strategies for patients with chronic wounds [2, 17].
In this review, we first discuss the undoubted benefits of the advances that have enabled assay of the peel microbiota and highlight the challenges of applying such research to the field of wound healing. Afterwards describing the results obtained and so far and presenting a forthcoming clinical application in patients with chronic wounds, we then provide updated information on the mechanisms by which the bacteria identified in these studies may contribute to delayed wound healing.
Skin Microbiota Analysis in Chronic Wounds: Advances and Challenges
Civilisation-based techniques have been used since the late 1800s as the method of selection to place leaner constituting the skin microbiota. Although these techniques were useful, they but immune identification of microorganisms that grew nether usual laboratory conditions, which were non necessarily the most prominent microorganisms [17]. Moreover, the civilisation of anaerobic bacteria is fastidious and usually challenging [17, 18]. Biases associated with culture-based approaches announced to have been overcome with the contempo development of next-generation sequencing targeting the species-specific small subunit ribosomal RNA (16S rRNA) cistron, a method that is at present widely accessible [17, eighteen]. This culture-independent molecular technique allows label of the wound microbiota according to all iii of the dimensions considered as essential for understanding its role in wound healing: microbial load, microbial diversity, and the presence of pathogens [17]. The 16S rRNA sequencing method has shown high sensitivity in wound microbiota analyses, revealing greater bacterial diversity and load than traditional culture-based methods [17,eighteen,19]. In a written report of the microbiome of diabetic foot ulcers, culture-based techniques underestimated bacterial load by ii.34 logs on boilerplate and by > half dozen logs in some cases, and besides failed to place an average of at least 26 bacterial species per diabetic pes ulcer when compared with 16S rRNA sequencing [eighteen]. As well, in some other study of chronic wounds of various etiologies, 17 unlike bacterial taxa were identified with aerobic cultures, whereas 338 different bacterial taxa were identified in the same samples with 16S rRNA sequencing [19]. Of note, nine of the xx well-nigh prominent bacteria identified with 16S rRNA sequencing in this study were anaerobes and would therefore not have been identified if aerobic cultures had been the only identification method used [19].
Although 16S rRNA sequencing addresses many of the limitations of culture-based approaches, it has its own limitations. Indeed, many leaner can be identified using molecular-based techniques, but the active contribution of these bacteria to wound outcomes is unknown [17, 18, twenty]. Furthermore, although sequencing may allow differences betwixt "healing-capable" and "healing-dumb" individuals to be identified, the result of correlation vs causation nonetheless remains. Gathering longitudinal data beyond multiple timepoints or assessing skin microbiota composition before disease manifestation or at the initial onset of a wound may help to observe causative links [21]. Moreover, complementary approaches, including metagenomic analyses, should be incorporated into studies of wound healing because 16S rRNA sequencing cannot identify nonbacterial microorganisms (e.k., fungi and viruses), which likewise constitute the skin microbiota and may influence wound healing outcomes [17, 18, 22]. The metagenomic approach (shotgun DNA sequencing) not just allows profiling of all microbial genomes in a sample, information technology besides allows functional and metabolic pathway analyses based on the sequences of Dna fragments throughout the genomes. Unlike 16S rRNA sequencing, the metagenomic approach provides identification of microbiome compositions at the strain-level resolution [21].
Beyond the methods used to investigate peel microbiota composition, further factors should be considered to attain high-quality, standardized skin microbiome research and enable reproducibility and comparisons betwixt studies. Ii recent studies [21, 23] identified potential pitfalls related to pare microbiome research, and established guidelines for conducting and reporting such investigations that encouraged consideration of the study blueprint, skin sample collection/storage, sample processing, sequencing approaches, required controls, analysis pipelines, and data deposition.
Microbiota Limerick of Chronic Wounds
Individuals at Hazard of Chronic Wound Development
Diabetic human foot ulcers, a common complication of diabetes, are a widespread type of chronic wound [2, 24]. To investigate whether changes in composition were nowadays in the skin microbiome of individuals at risk of developing these lesions, Redel et al. [25] conducted a case-control observational study using high-throughput 16S rRNA sequencing technology to analyze the pare microbiota from the arms and feet of diabetic men without any previous history of diabetic foot ulcers and from those of matched nondiabetic men as controls. Although microbiota composition and total bacterial counts were like in the arm samples of both groups, higher bacterial variety was observed in the plantar pes samples of diabetic men compared to nondiabetic men. The relative abundance of Firmicutes was lower in diabetic men after adjustment for the faux discovery charge per unit, whereas the abundance of Actinobacteria was constitute to be higher. Moreover, the plantar foot samples of diabetic men displayed a lower relative abundance of Staphylococcus species overall than those of nondiabetic men. However, a higher quantity of more virulent forms of Staphylococcus aureus characterized the foot microbiota in diabetic patients [25]. These skin microbiota differences between diabetic and nondiabetic individuals may be linked to the risk of futurity diabetic pes ulcer development [25]. Like studies are needed to investigate potential changes in the cutaneous microbiome in at risk populations, e.1000., patients with venous insufficiency at gamble of venous leg ulcer development and patients with spinal string injury at adventure of pressure ulcer evolution.
Individuals with Chronic Wounds
Initial studies examining microbiota in chronic wounds using 16S rRNA sequencing did not investigate correlations betwixt microbiota composition and wound outcomes. Based on a review of five microbiome studies, Misic et al. [17] plant most bacteria colonizing chronic wound tissue belonged to 21 families, among which Staphylococcaceae and Pseudomonadaceae were predominant regardless of the etiology of the wound and the blazon of sampling [18, 26,27,28,29]. Moreover, a large-scale retrospective study [20] of the microbiota from 2963 samples from chronic wounds of various etiologies showed that Staphylococcus and Pseudomonas were the virtually common genera, identified in 63% and 25% of the wounds, respectively. Staphylococcus aureus and S. epidermidis were the predominant species, and methicillin-resistant Staphylococcus species were identified in about 25% of the chronic wounds [20]. Of notation, commensal bacteria including Corynebacterium and Propionibacterium species, as well as anaerobic bacteria, were highly prevalent in the analyzed chronic wound samples [twenty]; however, the microbiota of chronic wounds is distinct from the surrounding healthy pare microbiota. No significant differences in microbiota limerick were observed across wound types [xx]. Additional smaller scale studies besides identified Due south. aureus every bit the nigh mutual species in chronic wounds of various etiologies [xix] and in neuropathic diabetic foot ulcers [18, 24]. In the report by Gardner et al. [eighteen], associations between microbiota composition and clinical factors were identified: loftier microbial variety, besides as increases in the relative abundance of anaerobic bacteria and Gram-negative Proteobacteria, were observed in deep ulcers and in those of long duration, whereas a great affluence of Staphylococcus, mainly Southward. aureus, was found in superficial ulcers and in those of brusque duration. The same group evaluated the temporal dynamics of the microbiota colonizing diabetic foot ulcers and found that rapid and dynamic changes in the microbiota were associated with faster healing and better outcomes [24]. Using shotgun metagenomics, Kalan et al. [16] identified strain-specific differences in the microbiome of diabetic foot ulcers that correlated with clinical outcomes of healing. Two clinical Southward. aureus isolates were found to exist associated with nonhealing wounds, SA10757 and SA7372, and healing inhibition was confirmed in mouse models. In contrast, wounds infected with Alcaligenes faecalis, a common ecology bacterium, healed at rates similar to controls [16]. Further investigation revealed that A. faecalis was associated with loftier levels of interleukin-eight and other cytokines that enhance wound healing [16]. These studies indicate that functional level differences betwixt microbiota species, or fifty-fifty between specific microbiota strains, may play an important role in determining the clinical outcomes of chronic wounds.
Structural Organization of the Skin Microbiota in Chronic Wounds: The Biofilm
Biofilm Clarification and Characteristics in Wounds
Microorganisms constituting the chronic wound microbiota accept been shown to be mainly organized in biofilms, i.east., a circuitous microbial customs containing bacteria and fungi surrounded by a polymeric matrix composed of polysaccharides, lipids, proteins, and nucleic acids [30, 31]. Using light and scanning electron microscopy techniques, James et al. [32] identified biofilms in 60% of chronic wounds simply in only six% of acute wounds. In a systematic review and meta-analysis gathering data from eight prospective cohort studies including 185 chronic wounds and ane case report series [33], the prevalence of biofilms in nonhealing man chronic wounds assessed by microcopy—associated in some cases with molecular methods—was 78.ii%, varying between 60 and 100%. Therefore, biofilms are suspected to contribute to chronic wound pathology and dumb wound healing.
Can Biofilms in Chronic Wounds be Used for Diagnostic Purposes?
Because of the predominance of biofilms in chronic wounds and the role of bacteria able to form biofilms in impaired wound healing (see Sect. four.i), a device or a method that could quickly detect the presence of biofilms in a wound would be useful for developing constructive treatment options and for monitoring treatment progress [31]. Schultz and Sampson [34] described one method for detecting biofilms based on staining with ruthenium red or Alcian bluish to reveal the mucopolysaccharides of the biofilm matrix [34]. In a prospective observational study testing the clinical applicability of this noninvasive method, Nakagami et al. [35] found that it could be used to predict changes in wound slough (biofilm) development in patients with pressure ulcers who had undergone precipitous wound debridement and wound washing before biofilm detection. In a more than recent retrospective observational written report [36], the aforementioned group demonstrated that this biofilm detection method could exist used to monitor the effect of biofilm emptying by sharp debridement: 1 calendar week after blotting, the percentage subtract in wound area was significantly college in the biofilm-eliminated group than in the biofilm-remaining group, and the presence of remaining biofilms was an contained predictive factor for lower percentage decreases in the wound area [36]. These results showed that biofilm removal using sharp debridement improved pressure ulcer healing. This quick and noninvasive method could therefore be useful for helping clinicians eliminate entire biofilms by detecting and mapping their distribution on the wound. A larger clinical trial is needed to validate this method for clinical employ.
Mechanisms by Which the Skin Microbiota Contribute to Delayed Wound Healing: Findings from Experimental Models
Extracellular Bacteria Found in Biofilms
Biofilms can develop from a unmarried bacterial or fungal species, can be polymicrobial (i.eastward., consist of several species), or tin fifty-fifty bridge kingdoms by being composed of interacting leaner and fungi [31]. Several studies take reported that most chronic nonhealing wounds are polymicrobial in nature, including a large-scale retrospective assay conducted by Wolcott et al. [20] in which 93% (northward = 2963) of samples from wounds of various etiologies were polymicrobial. Thus, investigating crosstalk amidst species is essential for understanding the regulation of wound healing. With this aim, our group used a well-established porcine wound model to report the interactions between two of the most prevalent bacterial species identified in both acute and chronic wounds of various etiologies: Southward. aureus and Pseudomonas aeruginosa [13]. Using methicillin-resistant S. aureus (MRSA) USA300-0114 and a P. aeruginosa clinical wound isolate, we showed that both species co-existed in porcine cutaneous wounds in vivo [13]. We also plant that MRSA did non touch on the growth of P. aeruginosa, but that P. aeruginosa reduced MRSA growth in the mixed-species biofilms both in vivo and in vitro, with a lower outcome observed in vivo than in vitro [13]. Moreover, the presence of P. aeruginosa in the co-infected wounds led to higher expression of ii MRSA virulence factors: Panton-Valentine leukocidin (pvl) at 2 and four days post-wounding, and α-hemolysin (Hla) at 4 days post-wounding [13]. Finally, nosotros showed that in comparison with uninfected wounds and wounds infected with single-species biofilms, wounds co-infected with P. aeruginosa and MRSA showed delayed epithelialization through down-regulation of keratinocyte growth cistron 1 expression [13]. Mainly produced by fibroblasts during the acute wound healing process, keratinocyte growth cistron 1 is known to play an important role in re-epithelialization past stimulating keratinocyte proliferation and migration in a paracrine manner [13]. In conclusion, we observed that interactions inside mixed-species biofilms resulted in a decreased load but increased virulence of MRSA, and significantly delayed wound healing in comparison with uninfected wounds and wounds infected with single-species biofilms [13]. Like findings from murine [37, 38] and rabbit [39] wound infection models underscore the contribution of polymicrobial wound environments to impaired host responses and inhibition of healing. Future studies utilizing metatranscriptomics (RNA transcript sequencing) with the ability to identify the full range of actively transcribed genes from both the host and the microbiome will enable in-depth analyses of host–microbe interactions in the wound environment.
Intracellular Bacterial Niche
Although biofilms play an of import role in the recurrent infections observed in patients with chronic wounds, other factors are involved in chronic wound infections. We hypothesized that, in addition to extracellular leaner, the intracellular microbial niche contributes to the damage of wound healing. Keratinocytes constitute the showtime cellular barrier to infection at the peel level. Similar innate allowed cells, they were recently shown to constitutively express Perforin-2 (P-two) in both mice and humans [forty]. Perforin-2 is an ancient innate immune protein that is highly conserved from sponges to humans [40, 41]. Perforin-2 is located within the membranes of endosomal vesicles, which traffic to and fuse with leaner-containing phagosomes thus enabling emptying of intracellular bacteria [xl, 42, 43]. Perforin-2 has been shown to be critical for the clearance of a variety of Gram-positive and Gram-negative pathogens, highlighting its pivotal role in the host'due south innate antimicrobial response [17].
To decide the bactericidal office of P-2 confronting MRSA in vivo, our group studied the effect of infecting P-2 scarce mice, heterozygous mice, and wild-type mice with a traditionally sub-lethal load of MRSA after record stripping [17]. Twelve days subsequently MRSA epicutaneous infection, MRSA could merely be recovered from the internal organs of a few heterozygous mice, whereas wild-type mice simply had recoverable colony-forming units in the skin. Yet, bacterial colony-forming units were recovered from the claret, spleen, and kidney of all P-ii-scarce mice. These animals failed to eliminate the MRSA and somewhen succumbed to infection. We have also investigated P-two expression and cellular distribution in human skin [44]. Using an amplified fluorescence in situ hybridization coupled with cell sorting (FISH-Catamenia) technique, nosotros found that P-ii transcripts were detected in gamma delta T cells, endothelial cells, keratinocytes, and fibroblasts, confirming P-2 expression past both professional and nonprofessional immune cells (Fig. 2). In improver, we showed that a keratinocyte prison cell line overexpressing a P-2-GFP fusion protein cleared intracellular S. aureus infection more efficiently than control cells, supporting our findings of the importance of P-2 in bacterial clearance in the skin [44]. We also used an ex vivo human wound model to evaluate P-2 expression during wound healing and to determine how levels alter in response to the most common pare and wound pathogen, S. aureus. We found that although P-2 expression was induced during wound healing, S. aureus infection inhibited P-ii expression in both hematopoietic and nonhematopoietic cells resulting in inhibition of wound closure (Fig. 2) [44].
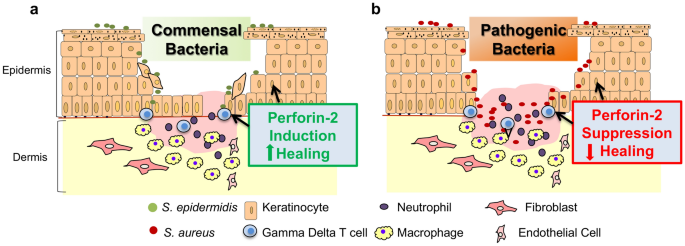
Cutaneous amnesty is differentially regulated past commensal and pathogenic microorganisms through modulation of Perforin-ii. a Colonization of the wound with commensal bacteria may promote wound healing by inducing antimicrobial proteins such every bit Perforin-2, thus stimulating a protective immune response against pathogenic bacteria. b Wound infection with pathogenic bacteria results in Perforin-two suppression in both hematopoietic and nonhematopoietic cells and inhibition of healing
Conversely, peel commensals, including South. epidermidis, have been found to promote healing and to induce expression of different antimicrobials [14]. Given the prevalence of Due south. aureus in chronic wounds, it seems likely that S. aureus suppression of P-two promotes bacterial persistence at the wound site resulting in wound chronicity. Conversely, the presence of commensal leaner may modify the pare and wound surroundings to forbid or resolve bacterial wound infections. Further studies on the mechanisms of P-two action have the potential to provide major advances in our understanding of innate amnesty in the skin, and the insights gained could exist applied to diseases that compromise the skin barrier without causing chronic wounds, such as atopic dermatitis.
Conclusions and Perspectives
Recent advances have enabled improve characterization of leaner in chronic wounds. More importantly, the latest studies did not only draw "what is there?", just besides established correlations with wound healing outcomes and provided insights on "what is it doing?". This is the area of agile investigation that continuously reveals new potential pathways for therapeutic intervention for patients with chronic wounds. Microorganisms constituting "the chronic wound microbiota" are mainly organized in the course of a polymicrobial biofilm. Interactions between dissimilar species within the polymicrobial environment have been shown to exist dynamic and to modify bacterial behavior, resulting in increased virulence and delayed wound healing. It is necessary to further investigate such microbiome-host interactions to place new potential treatments. Indeed, our ain research showed that antibiotic-resistant leaner can subvert P-2 to prevent them from existence killed. Understanding how one of the most frequent wound colonizers, S. aureus, evades the antimicrobial activity of P-2, too as how commensal leaner may prevent or opposite the inhibitory event of pathogens, volition provide primal new insights into the intricate mechanisms by which commensal and pathogenic bacteria mediate crosstalk with the cutaneous immune system during wound healing. Understanding the mechanisms of P-ii activation volition enable the development of therapies to clear antibiotic-resistant chronic wound pathogens. It would too be interesting to assess whether molecular analyses of chronic wound microbiota could be used to determine treatment efficacy and heighten the development of new therapies. Because of the complication and dynamics of the cutaneous wound healing procedure, the development of combination treatments—targeting both the host and the microbiome—could be necessary to augment healing and prevent and treat infections in wounds, peel, and soft tissues.
References
-
Gould L, Abadir P, Brem H, Carter Chiliad, Conner-Kerr T, Davidson J, et al. Chronic wound repair and healing in older adults: current condition and hereafter research. Wound Repair Regen. 2015;23(ane):1–13.
-
Eming SA, Martin P, Tomic-Canic Thou. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. 2014;six(265):265sr6.
-
Fayne RA, Borda LJ, Egger AN, Tomic-Canic G. The potential bear on of social genomics on wound healing. Adv Wound Care (New Rochelle). 2020;9(6):325–31.
-
Moulik PK, Mtonga R, Gill GV. Amputation and mortality in new-onset diabetic pes ulcers stratified by etiology. Diabetes Care. 2003;26(2):491–4.
-
Armstrong DG, Wrobel J, Robbins JM. Guest editorial: are diabetes-related wounds and amputations worse than cancer? Int Wound J. 2007;4(four):286–7.
-
Nussbaum SR, Carter MJ, Fife CE, DaVanzo J, Haught R, Nusgart M, et al. An economic evaluation of the touch, price, and Medicare policy implications of chronic nonhealing wounds. Value Health. 2018;21(1):27–32.
-
Ramirez HA, Pastar I, Jozic I, Stojadinovic O, Stone RC, Ojeh N, et al. Staphylococcus aureus triggers induction of miR-15B-5P to diminish DNA repair and deregulate inflammatory response in diabetic foot ulcers. J Invest Dermatol. 2018;138(5):1187–96.
-
Stone RC, Stojadinovic O, Rosa AM, Ramirez HA, Badiavas E, Blumenberg M, et al. A bioengineered living jail cell construct activates an acute wound healing response in venous leg ulcers. Sci Transl Med. 2017;ix(371):eaaf8611.
-
Stone RC, Stojadinovic O, Sawaya AP, Glinos GD, Lindley LE, Pastar I, et al. A bioengineered living jail cell construct activates metallothionein/zinc/MMP8 and inhibits TGFbeta to stimulate remodeling of fibrotic venous leg ulcers. Wound Repair Regen. 2020;28(2):164–76.
-
Pastar I, Wong LL, Egger AN, Tomic-Canic G. Descriptive vs mechanistic scientific approach to written report wound healing and its inhibition: is in that location a value of translational research involving homo subjects? Exp Dermatol. 2018;27(5):551–62.
-
Thom SR, Hampton Thousand, Troiano MA, Mirza Z, Malay DS, Shannon South, et al. Measurements of CD34+/CD45-dim stem cells predict healing of diabetic neuropathic wounds. Diabetes. 2016;65(ii):486–97.
-
Zeeuwen PL, Boekhorst J, van den Bogaard EH, de Koning HD, van de Kerkhof PM, Saulnier DM, et al. Microbiome dynamics of human epidermis following pare barrier disruption. Genome Biol. 2012;13(xi):R101.
-
Pastar I, Nusbaum AG, Gil J, Patel SB, Chen J, Valdes J, et al. Interactions of methicillin resistant Staphylococcus aureus USA300 and Pseudomonas aeruginosa in polymicrobial wound infection. PLoS One. 2013;8(2):e56846.
-
Harrison OJ, Linehan JL, Shih HY, Bouladoux Northward, Han SJ, Smelkinson G, et al. Commensal-specific T cell plasticity promotes rapid tissue adaptation to injury. Science. 2019;363(6422):eaat6280.
-
Lai Y, Di Nardo A, Nakatsuji T, Leichtle A, Yang Y, Cogen AL, et al. Commensal bacteria regulate Toll-like receptor 3-dependent inflammation after skin injury. Nat Med. 2009;fifteen(12):1377–82.
-
Kalan LR, Meisel JS, Loesche MA, Horwinski J, Soaita I, Chen 10, et al. Strain- and species-level variation in the microbiome of diabetic wounds is associated with clinical outcomes and therapeutic efficacy. Cell Host Microbe. 2019;25(5):641–55.e5.
-
Misic AM, Gardner SE, Grice EA. The wound microbiome: modernistic approaches to examining the role of microorganisms in dumb chronic wound healing. Adv Wound Care (New Rochelle). 2014;3(seven):502–ten.
-
Gardner SE, Hillis SL, Heilmann M, Segre JA, Grice EA. The neuropathic diabetic foot ulcer microbiome is associated with clinical factors. Diabetes. 2013;62(3):923–xxx.
-
Rhoads DD, Wolcott RD, Dominicus Y, Dowd SE. Comparison of culture and molecular identification of bacteria in chronic wounds. Int J Mol Sci. 2012;13(three):2535–50.
-
Wolcott RD, Hanson JD, Rees EJ, Koenig LD, Phillips CD, Wolcott RA, et al. Analysis of the chronic wound microbiota of 2963 patients by 16S rDNA pyrosequencing. Wound Repair Regen. 2016;24(1):163–74.
-
Kong HH, Andersson B, Clavel T, Mutual JE, Jackson SA, Olson ND, et al. Performing skin microbiome research: a method to the madness. J Invest Dermatol. 2017;137(3):561–eight.
-
Kalan Fifty, Loesche M, Hodkinson BP, Heilmann G, Ruthel Yard, Gardner SE, et al. Redefining the chronic-wound microbiome: fungal communities are prevalent, dynamic, and associated with delayed healing. mBio. 2016;7(v):e01058–116.
-
Grogan MD, Bartow-McKenney C, Flowers 50, Knight SAB, Uberoi A, Grice EA. Research techniques fabricated elementary: profiling the pare microbiota. J Invest Dermatol. 2019;139(4):747–52.e1.
-
Loesche Thousand, Gardner SE, Kalan L, Horwinski J, Zheng Q, Hodkinson BP, et al. Temporal stability in chronic wound microbiota is associated with poor healing. J Invest Dermatol. 2017;137(ane):237–44.
-
Redel H, Gao Z, Li H, Alekseyenko AV, Zhou Y, Perez-Perez GI, et al. Quantitation and composition of cutaneous microbiota in diabetic and nondiabetic men. J Infect Dis. 2013;207(seven):1105–14.
-
Price LB, Liu CM, Frankel YM, Melendez JH, Aziz M, Buchhagen J, et al. Macroscale spatial variation in chronic wound microbiota: a cantankerous-sectional written report. Wound Repair Regen. 2011;19(1):lxxx–eight.
-
Price LB, Liu CM, Melendez JH, Frankel YM, Engelthaler D, Aziz Thousand, et al. Community analysis of chronic wound bacteria using 16S rRNA factor-based pyrosequencing: touch on of diabetes and antibiotics on chronic wound microbiota. PLoS One. 2009;four(7):e6462.
-
Wolcott RD, Gontcharova V, Sunday Y, Dowd SE. Evaluation of the bacterial diversity among and within individual venous leg ulcers using bacterial tag-encoded FLX and titanium amplicon pyrosequencing and metagenomic approaches. BMC Microbiol. 2009;27(9):226.
-
Frank DN, Wysocki A, Specht-Glick DD, Rooney A, Feldman RA, St Amand AL, et al. Microbial diversity in chronic open up wounds. Wound Repair Regen. 2009;17(ii):163–72.
-
Monroe D. Looking for chinks in the armor of bacterial biofilms. PLoS Biol. 2007;v(11):e307.
-
Phillips PL, Wolcott RD, Fletcher J, Schultz GS. Biofilms made easy. Wounds Int. 2010;1(3):1–6.
-
James GA, Swogger Eastward, Wolcott R, Pulcini E, Secor P, Sestrich J, et al. Biofilms in chronic wounds. Wound Repair Regen. 2008;xvi(ane):37–44.
-
Malone M, Bjarnsholt T, McBain AJ, James GA, Stoodley P, Leaper D, et al. The prevalence of biofilms in chronic wounds: a systematic review and meta-analysis of published data. J Wound Intendance. 2017;26(i):20–five.
-
Schultz GS, Phillips PL, Sampson EM. Materials and methods for assessing and mapping microbes and microbial biofilms on wound. US Patent Application Publication, Pub No.: Us 2012/0322048 A1; 2012.
-
Nakagami Thousand, Schultz Thousand, Gibson DJ, Phillips P, Kitamura A, Minematsu T, et al. Biofilm detection by wound blotting can predict slough development in force per unit area ulcers: a prospective observational study. Wound Repair Regen. 2017;25(i):131–eight.
-
Nakagami One thousand, Schultz Thousand, Kitamura A, Minematsu T, Akamata K, Suga H, et al. Rapid detection of biofilm past wound blotting post-obit sharp debridement of chronic pressure level ulcers predicts wound healing: a preliminary written report. Int Wound J. 2020;17(1):191–six.
-
Dalton T, Dowd SE, Wolcott RD, Lord's day Y, Watters C, Griswold JA, et al. An in vivo polymicrobial biofilm wound infection model to study interspecies interactions. PLoS One. 2011;6(11):e27317.
-
Dhall Southward, Do D, Garcia M, Wijesinghe DS, Brandon A, Kim J, et al. A novel model of chronic wounds: importance of redox imbalance and biofilm-forming bacteria for institution of chronicity. PLoS One. 2014;9(10):e109848.
-
Seth AK, Geringer MR, Hong SJ, Leung KP, Galiano RD, Mustoe TA. Comparative analysis of unmarried-species and polybacterial wound biofilms using a quantitative, in vivo, rabbit ear model. PLoS One. 2012;7(8):e42897.
-
McCormack RM, de Armas LR, Shiratsuchi Chiliad, Fiorentino DG, Olsson ML, Lichtenheld MG, et al. Perforin-ii is essential for intracellular defense of parenchymal cells and phagocytes against pathogenic bacteria. Elife. 2015;24(4):e06508.
-
McCormack R, Podack ER. Perforin-ii/Mpeg1 and other pore-forming proteins throughout evolution. J Leukoc Biol. 2015;98(v):761–eight.
-
Pang SS, Bayly-Jones C, Radjainia One thousand, Spicer BA, Law RHP, Hodel AW, et al. The cryo-EM structure of the acid activatable pore-forming immune effector macrophage-expressed factor 1. Nat Commun. 2019;x(ane):4288.
-
Ni T, Jiao F, Yu X, Aden S, Ginger Fifty, Williams SI, et al. Construction and machinery of bactericidal mammalian perforin-ii, an ancient agent of innate immunity. Sci Adv. 2020;6(five):eaax8286.
-
Strbo Northward, Pastar I, Romero L, Chen Five, Vujanac M, Sawaya AP, et al. Single cell analyses reveal specific distribution of anti-bacterial molecule Perforin-2 in human pare and its modulation by wounding and Staphylococcus aureus infection. Exp Dermatol. 2019;28(3):225–32.
Acknowledgements
The authors thank Laurence Rous, PhD, Emma Pilling, PhD, and Marielle Romet, PhD (Synergy Pharm-Santé Active Edition) for medical writing aid funded by Laboratoires Dermatologiques Avène, Pierre Fabre Dermo-Cosmétique.
Author data
Affiliations
Corresponding author
Ideals declarations
Funding
Medical writing assist was funded by Laboratoires Dermatologiques Avène, Pierre Fabre Dermo-Cosmétique. The work presented was in part funded by the National Institutes of Health, National Establish for Nursing Research (NR015649).
Disharmonize of interest
Marjana Tomic-Canic, Jamie 50. Burgess, Katelyn E. O'Neill, Natasa Strbo, and Irena Pastar have no conflicts of interest that are directly relevant to the content of this article.
Disclosure
This commodity is published equally part of a journal supplement wholly funded by Laboratoires Dermatologiques Avène, Pierre Fabre Dermo-Cosmétique.
Author Contributions
MTC, NS, and IP: contributed to the conception of the manuscript; IP, MTC, JLB, KO, and NS: performed the literature search and data analysis, and critically revised the piece of work. All authors edited the terminal draft.
Rights and permissions
Open up Access This article is licensed under a Creative Eatables Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, accommodation, distribution and reproduction in any medium or format, every bit long as you give advisable credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were fabricated. The images or other third party material in this article are included in the article's Creative Eatables licence, unless indicated otherwise in a credit line to the material. If material is non included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, yous will need to obtain permission direct from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/past-nc/four.0/.
Reprints and Permissions
Nearly this article
Cite this article
Tomic-Canic, K., Burgess, J.L., O'Neill, K.E. et al. Skin Microbiota and its Interplay with Wound Healing. Am J Clin Dermatol 21, 36–43 (2020). https://doi.org/10.1007/s40257-020-00536-w
-
Published:
-
Effect Engagement:
-
DOI : https://doi.org/10.1007/s40257-020-00536-westward
Source: https://link.springer.com/article/10.1007/s40257-020-00536-w
Posted by: palaciosspold1973.blogspot.com


0 Response to "Which Of These Would Not Describe Characteristics Of The Normal Microbiota Of The Skin?"
Post a Comment